Drugs Information:
Dulaglutide
Basic Information
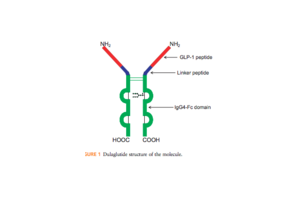
|
||
| ID | DDInter607 | |
| Drug Type | biotech | |
| Protein Chemical Formula | C2646H4044N704O836S18 | |
| Protein Average Weight | 59669.810 | |
| CAS Number | 923950-08-7 | |
| Description | Dulaglutide, marketed by Eli Lilly as Trulicity, is a once-weekly subcutaneous glucagon-like peptide-1 (GLP-1) receptor agonist designed using recombinant DNA technology; it has been approved as an adjunct therapy to diet and exercise in the management of 2 diabetes (T2DM).[A236070] Dulaglutide was initially approved by the FDA in 2014, and in February 2020 was approved for use in patients with T2DM and multiple cardiovascular risk factors for the prevention of cardiovascular events. It is the first T2DM drug approved to reduce major adverse cardiovascular events (MACE) risk in primary and secondary prevention populations.[L34665] | |
| ATC Classification | A10BJ05 | |
| Sequences | > Dulaglutide Sequence HGEGTFTSDVSSYLEEQAAKEFIAWLVKGGGGGGGSGGGGSGGGGSAESKYGPPCPPCPA PEAAGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSQEDPEVQFNWYVDGVEVHNAKTKP REEQFNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKGLPSSIEKTISKAKGQPREPQVYTL PPSQEEMTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSRLT VDKSRWQEGNVFSCSVMHEALHNHYTQKSLSLSLG | |
| Useful Links | DrugBank PubChem Substance KEGG Drug UniProtKB Wikipedia ChEMBL | |
Interactions with
Dulaglutide
Filter:
| Severity level | ID | Name | Mechanism | Detail |
|---|
Interactions with diseases
Filter:
| Severity level | Disease name | Text | References |
|---|
Interactions with foods
Filter:
| Severity level | Food name | Description | Management | Mechanism | References |
|---|
Interactions with compound preparation
| Multi-DRUG trade | Multi-DRUG | Drug type | Warning | Note |
|---|